5. Citric acid is the main acid present in lemon juice. However vitamin C, or ascorbic acid is
also present. Ascorbic acid also reacts with baking soda to produce carbon dioxide. Does
the
presence
of ascorbic acid mean the concentration of citric acid you calculated is lower
than the true value or higher? Why?
Answers
In the titration of lemon juice, the presence of ascorbic acid means the concentration of citric acid you calculated is higher.
An acid-base titration is a common way to determine the unknown concentration of an acid, given we know the concentration of the base and determine the spent volume in the titration. Let's consider the neutralization reactions that take place in a mixture of citric acid and ascorbic acid.
Citric acid titration :
3 NaOH(aq) + H₃C₆H₅O₇(aq) → Na₃C₆H₅O₇(aq) + 3 H₂O(l)
Ascorbic acid titration:
NaOH(aq) + HC₆H₇O₆(aq) → NaC₆H₇O₆(aq) + H₂O(l)
If we titrated a solution that contained only citric acid, we can relate through stoichiometry the moles and concentration of citric acid. However, if the solution also contained ascorbic acid, we would have to spend more NaOH to titrate it. Since more NaOH would react, we would conclude that there is more citric acid to react, calculating a higher concentration of the same.
In the titration of lemon juice, the presence of ascorbic acid means the concentration of citric acid you calculated is higher.
You can learn more about titration here: https://brainly.com/question/2728613

Related Questions
Question 16 of 30
Which substance will form a solution when mixed with solid sodium chloride?
O A. CCl4(0)
SUBMIT
B. CH₂OH()
C. Cl₂(g)
OD. NaO₂ (s)
Answers
Answer: B. CH₂OH
Explanation:
First off, we need a liquid solvent. Eliminate C and D.
Now, we have to consider polarity.
Carbon tetrachloride is nonpolar, so sodium chloride will be insoluble.CH₂OH is polar, so sodium chloride will be soluble.
What is the ratio of the volume of oxygen gas to the
volume of water vapor in the following reaction?
Answers
Answer:
The ratio of volumes in the given case illustrates the Gay Lussac's Law of Gaseous Volumes.
- The law was given by the french chemist Gay Lussac in the year 1806.
- He stated that when gases are combined or are produced in a chemical reaction, they do so in a simple ratio by volume.
- It is given that 2 volumes of hydrogen combine with 1 volume of oxygen producing 2 volumes of water.
- This is in accordance with this law.
Explanation:
PLEASE HELP QUICKLY!!!
HI gas is removed from the system
at equilibrium below. How does the
system adjust to reestablish
equilibrium?
51.8 kJ + H₂(g) + 1₂(g) = 2HI(g)
A. The reaction shifts to the right (products) and the concentrations
of I, and H₂ decrease.
B. The reaction shifts to the left (reactants) and the concentrations
of H₂ and I increase.
C. The reaction shifts to the right (products) and the concentrations
of I, and H₂ increase.
D. The reaction shifts to the left (reactants) and the concentration of
HI increases.

Answers
Answer:
A. The reaction shifts to the right (products) and the concentrations of I and H₂ decrease.
Explanation:
If gas is removed from the system at equilibrium, the system will try to compensate for the loss by shifting the reaction in a direction that produces more gas molecules. This is known as Le Chatelier's principle, which states that a system at equilibrium will respond to a disturbance by shifting in a way that minimizes the effect of the disturbance.
In this case, since gas is being removed from the system, the reaction will shift to the side that produces more gas molecules. Looking at the balanced equation, we can see that 2HI(g) has a greater number of gas molecules compared to H₂(g) and I₂(g). Therefore, the system will shift to the right (products) to produce more HI(g) and reestablish equilibrium.
What are the answers to the following 5 multiple choice questions

Answers
The balanced equation of the reaction is option d. The limiting reactant here is KBr and excess reactant is calcium nitrate. The percent yield of the reaction is 93 %.
What is limiting reactant ?The limiting reactant in a reaction is the reactant which is fewer in amount and as soon it is consumed, the reaction stops. For the given reaction, option d is the balanced chemical equation.
One mole of calcium nitrate requires 2 moles of KBr.
molar mass of calcium nitrate = 164 g/mol
no.of moles in 75 g = 75/164 = 0.457
molar mass of KBr = 118.9 g/mol
no.of moles 95 g = 95/118.9 = 0.798
0.457 moles of calcium nitrate needs its twice amount that is 0.9 moles of KBr. Hence, KBr is the limiting reactant and calcium nitrate is excess reactant here.
2 moles or 237.8 g of KBr gives 2 moles or 202 g of potassium nitrate. Then, 95 g of KBr will gives:
(95×202)/237.6 = 81.3 g
actual yield = 75.75 g
then percent yield = 75.75 /81.3 × 100 = 93 %.
0.79 moles of KBr needs its half or 0.399 moles of calcium nitrate. But we have 0.45 moles. Thus, excess amount is
(0.45 - 0.399) × 164 g/mol = 9.5 g.
Therefore, the 9.5 g of excess reactant will be left over.
Find more on limiting reactants:
https://brainly.com/question/28938721
#SPJ1
1. What are the two effects of the continental plate/continental plate convergent boundary?
2. Where can you find an example of this type of divergent boundary? Which country, continent or ocean?

Answers
Answer
oh you again?
Explanation:
Please help and explain thank you

Answers
The names of the compounds are;
1) Sodium carbonate
2) potassium hydroxide
3) Copper II nitrate
4) Iron II sulfate
5) Silver acetate
6) Lead II chromate
7) Manganese II chlorate
8) Iron II sulfite
9) Nickel III phosphate
10) Chromium III bicarbonate
What are polyatomic ionic compound?An example of a polyatomic ionic molecule is one that has several atoms covalently bound together, carrying a net charge as a group, and acting as a single unit during chemical reactions.
These compounds contain polyatomic ions, charged entities made up of two or more atoms joined by covalent bonds but carrying an overall charge as a result of electron gain or loss.
Learn more about ionic compounds:https://brainly.com/question/9167977
#SPJ1
An ideal gas is confined to a 10.0-L balloon at STP. What is
the new volume of the balloon when it is placed under
800. mmHg at 100°C?
Answers
Answer:
\(V_2= 13.0L\)
Explanation:
Hello.
In this case, we can consider this problem related to ideal gases which are those that are not attracted or repulsed to each other, and can be studied via the ideal gas equation:
\(PV=nRT\)
Thus, since we have two conditions and the moles of the gas have not changed, we can write:
\(\frac{P_1V_1}{T_1}= \frac{P_2V_2}{T_2}\)
Because the initial conditions for 10.0 L of the gas are 1 atm and 273.15 K (STP) and the final conditions are 800 mmHg (1.053 atm), so the new volume is:
\(V_2=\frac{P_1V_1T_2}{T_1P_2}=\frac{1atm*10.0L*373.15K}{273.15K*1.053atm}\\\\V_2= 13.0L\)
Best regards!
When the balloon placed under 800. mmHg, the new volume will be "13.0 L".
Ideal gas equationAccording to the question,
Pressure, P₁ = 1 atm
P₂ = 1.053 atm
Temperature, T₁ = 273.15 K
T₂ = 373.15 K
Volume, V₁ = 10.0 L
By using the Ideal gas equation,
→ PV = nRT
or,
→ \(\frac{P_1 V_1}{T_2} = \frac{P_2 V_2}{T_2}\)
Then,
The new volume be:
→ V₂ = \(\frac{P_1 V_1 T_2}{T_1 P_2}\)
By substituting the above values,
= \(\frac{1\times 10.0\times 373.15}{273.15\times 1.053}\)
= 13.0 L
Thus the approach above is right.
Find out more information about ideal gas equation here:
https://brainly.com/question/20348074
How does hydrogen bonding affect solubility?
Answers
2. Polar compounds can form hydrogen bonds with water which lets them to dissolve in water.
3. Non-polar compounds cannot form hydrogen bonds, so they have lower solubility in water.
Carbonyl fluoride, cof2 , is an important intermediate used in the production of fluorine-containing compounds. For instance, it is used to make the refrigerant carbon tetrafluoride, cf4 via the reaction.
Answers
The concentration of carbonyl fluoride (COF2) remains at equilibrium is 0.33M if only COF2 is present initially at a concentration of 2.00 M.
The reaction given is an equilibrium reaction, and so the reaction can be expressed in terms of the equilibrium constant (Kc).
The equilibrium constant (Kc) for the reaction is given as = 5.10.
\(2COF2(g) ,--- > CO2(g)+CF4(g)\)
For an equilibrium reaction, the equilibrium concentration of the reactants can be determined using the following equation:
\(Kc = [CO2][CF4]/[COF2]^2\) Where [CO2], [CF4], and [COF2] are the equilibrium concentrations of the respective species.
Rearranging the equation gives us the following expression:
\([COF2]^2 = [CO2][CF4]/Kc\)
ICE chart is :
\(2COF2(g) < -- > CO2(g) + CF(g)\)
I 2 0 0
C -2x +x +x
----------------------------------------------------------
E 2-2x x x
Plugging in the values given, we can calculate the equilibrium concentration of COF2:
\(Kc = (x)(x)/(2-2x)^2\)
\(6.40 = x^2/(2-2x)^2\)
Taking the square root of both sides, we can calculate the equilibrium concentration of COF2:
x = 0.835M
[COF2] = 2-2x =2 -2(0.835) = 0.33M
Therefore, if only COF2 is present initially at a concentration of 2.00 M, the concentration of COF2 at equilibrium will be 0.33 M.
To learn more about Carbonyl fluoride click here https://brainly.com/question/4796698
#SPJ4
complete question: Carbonyl fluoride, COF2 , is an important intermediate used in the production of fluorine-containing compounds. For instance, it is used to make the refrigerant carbon tetrafluoride, CF4 via the reaction 2COF2(g)⇌CO2(g)+CF4(g), Kc = 6.40 . If only COF2 is present initially at a concentration of 2.00 M, what concentration of COF2 remains at equilibrium?
PLEASE HELP!!
Solutions Pre-Lab Questions:
In this lab, you will make fruit drinks with powdered drink mix. Complete the pre-lab questions to get the values you need for your drink solutions.
1. Calculate the molar mass of powered fruit drink mix, made from sucrose (C12H22O11).
2. Using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 M solution of 100 mL.
(Hint: Use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)
3. What mass of powdered drink mix is needed to make a 0.5 M solution of 100 mL?
Answers
Answer:
Explanation:
C₁₂H₂₂O₁₁
1 )
Molar mass = 12 x 12 + 22 x 1 + 11 x 16
= 144 + 22 + 176
= 342 g
2 )
100 mL of 1.0 M will contain 1.0 x0.100 = .1 mole of sucrose
0.1 mole of sucrose = 0.1 x 342 g = 34.2 g of sucrose.
So , mass of sucrose required is 34.2 g .
3 )
100 mL of .5 M sucrose = .100 x .5 mole of sucrose
= .05 mole of sucrose
.05 mole of sucrose = .05 x 342 g = 17.1 g of sucrose .
So , mass of sucrose required is 17.1 g .
Gauging yourself against others is acceptable in competitive sports.
Answers
Answer:
Yup it is acceptable.
U comparing yourself will place u above and below some people for sure.
So you will know to where you have to develop and can get yourself satisfied as you will be above some people.
Gauging yourself against others is acceptable in competitive sports is acceptable.
What is Competitive sports?Faster, higher, stronger is the Olympic motto frequently used in competitive sports. The Olympic slogan "To be there is everything" causes confusion, although it is actually highly false and the complete reverse.
Competitive athletes receive a planned training program. To attain particular objectives, the quantity and quality of each individual training block are carefully calculated.
The scope, duration, number, and load of each individual training unit are adjusted according to the level of fitness and the proximity to the season's peak throughout the year.
Therefore, Gauging yourself against others is acceptable in competitive sports is acceptable.
To learn more about competitive sports, refer to the link:
https://brainly.com/question/28193168
#SPJ5
What are the similarities and differences between ionic and covalent bonds?
Answers
Answer:
Explanation:
Similarities.
Both Ionic and covalents bond produce exothermic reactions.
They are both neutral.in Ionic bonds, the two opposite charge will terminate each other and in covalent, the neutral molecules tend to share electrons.
Difference
Ionic bonds have high polarity while covalent have low.
Ionic bonds have no definite shape, covalent have.
Ionic have high melting points, covalent have low.
Io ic have high boiling point, covalents have low.
HQ5.40
Homework Answered Due Today, 11:59 PM
The reaction 3H₂(g) + N₂(g) → 2NH3(g) has an enthalpy of reaction of -92.6 kJ/mol. If 1 g of hydrogen and 2 g of nitrogen are
reacted, how much heat is produced (kJ)?
Answers
The amount of heat energy produced when 1 g of hydrogen and 2 g of nitrogen are reacted, is -6.61 KJ
How do i determine the heat energy produced?First, we shall obtain the limiting reactant. Details below:
3H₂ + N₂ -> 2NH₃
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 g Molar mass of H₂ = 2 g/molMass of H₂ from the balanced equation = 3 × 2 = 6 gFrom the balanced equation above,
28 g of N₂ reacted with 6 g of H₂
Therefore,
2 g of N₂ will react with = (2 × 6) / 28 = 0.43 g of H₂
We can see that only 0.43 g of H₂ is needed in the reaction.
Thus, the limiting reactant is N₂
Finally, we the amount of heat energy produced. Details below:
3H₂ + N₂ -> 2NH₃ ΔH = -92.6 KJ
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 gFrom the balanced equation above,
When 28 grams of N₂ reacted, -92.6 KJ of heat energy were produced.
Therefore,
When 2 grams of N₂ will react to produce = (2 × -92.6) / 28 = -6.61 KJ
Thus the heat energy produced from the reaction is -6.61 KJ
Learn more about heat energy:
https://brainly.com/question/31429264
#SPJ1
A student titrated a solution containing 3.7066 g of an unknown Diprotic acid to the end point using 28.94 ml of 0.3021 M KOH solution. What is the molar mass of the unknown acid? Hint: you must write a balanced equation for the reaction.
Answers
Answer:
Molar mass of the diprotic acid is 424 grams
Explanation:
[hint: diprotic acid only contains 2 hydrogen protons]
Ionic equation:
\({ \bf{2OH { }^{ - } _{(aq)} + 2H { }^{ + } _{(aq)}→ 2H _{2} O _{(l)} }}\)
first, we get moles of potassium hydroxide in 28.94 ml :
\({ \sf{1 \: l \: of \: KOH \: contains \: 0.3021 \: moles}} \\ { \sf{0.02894 \: l \: of \: KOH \: contain \: (0.02894 \times 0.3021) \: moles}} \\ { \underline{ = 0.008743 \: moles}}\)
since mole ratio of diprotic acid : base is 2 : 2, moles are the same.
Therefore, moles of acid that reacted are 0.008743 moles.
\({ \sf{0.008743 \: moles \: of \: acid \: weigh \: 3.7066 \: g}} \\ { \sf{1 \: mole \: of \: acid \: weighs \: ( \frac{1 \times 3.7066}{0.008743}) \: g }} \\ = { \underline{423.95 \: g \approx424 \: grams}}\)
for the molar mass:
how are ionic bonds and covalent bonds different
Answers
Which of these waves has the greatest wavelength? (3 points) Wave shown with 2 wavelengths. Wave shown with 3 wavelengths. Wave shown with 1 wavelength stretch over a short distance. Wavelength shown with 1 wavelength stretched over a long distance.
Answers
The waves that has the greatest wavelength is Wavelength shown with 1 wavelength stretched over a long distance.
Waves explained.A wave could be a disturbance or variety that voyages through a medium or space, carrying vitality without transporting matter. Waves can take different shapes and happen totally different sorts of waves, counting mechanical waves and electromagnetic waves.
Mechanical waves require a medium to propagate, meaning they require a substance like water, discuss, or a strong fabric to transmit the wave. Illustrations of mechanical waves incorporate water waves, sound waves, and seismic waves. In these waves, particles of the medium sway or vibrate in a design, exchanging energy from one molecule to another.
Electromagnetic waves, on the other hand, don't require a medium and can travel through vacuum, such as in space. Electromagnetic waves comprise of electric and attractive areas swaying opposite to each other and to the heading of wave engendering. Illustrations of electromagnetic waves incorporate obvious light, radio waves, microwaves, infrared waves, bright waves, X-rays, and gamma beams.
Learn more about waves below.
https://brainly.com/question/26116832
#SPJ1
What is the length of the paper clip in cm
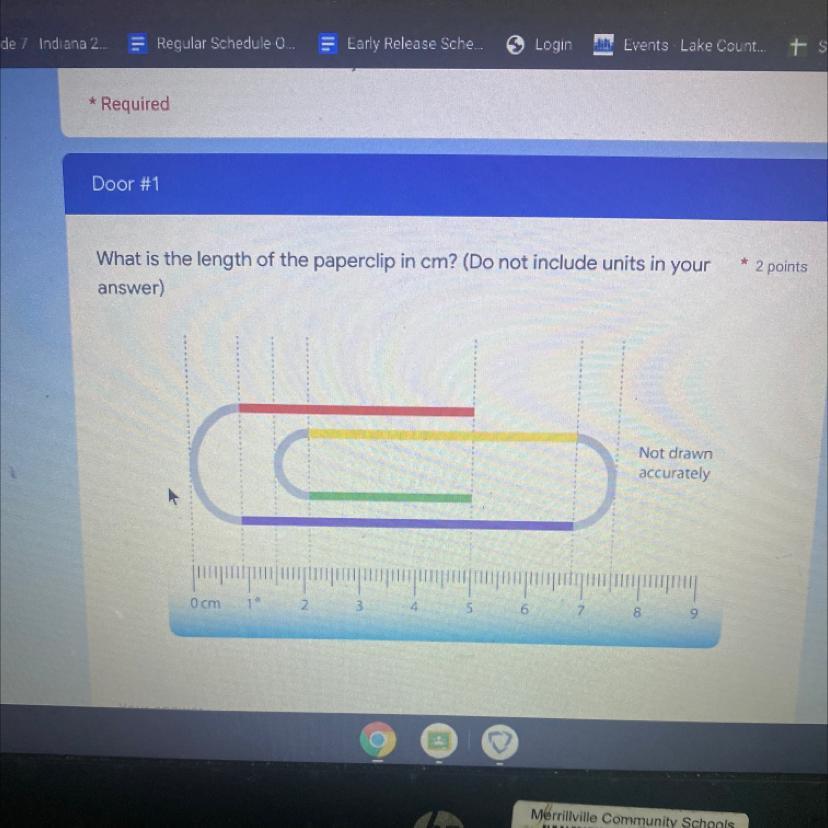
Answers
Answer:
24.5 cm
Explanation:
It would be really complicated to type out, so I've attached an image of how I solved this:
*I separated the paperclip into different sections, figured out the length of those sections, and added them together.
(sorry that my work isn't the neatest)

Using what you know about pneumatic lifts and gases, what do you think would happen to a pneumatic lift on a cold night in Chicago
Answers
For pneumatic elevators operating on cold Chicago nights, lifting capacity may be reduced due to the reduced pressure of the gas contained in the elevator. The exact extent of this drop will depend on the specific conditions of the elevator and the severity of the low temperature.
How much does an airlift system cost?
The average cost of an air suspension system ranges from $500 to over $2,000 depending on vehicle and location. If you just put an auxiliary air suspension spring on your truck, you can consider yourself on the lower end of the cost spectrum.
How long does the airlift take?
eternally! With proper installation and maintenance, our air springs will last indefinitely. Check pressure regularly or use the onboard air system to maintain it. Air Lift offers a lifetime warranty on all air spring kits.
To know more about pressure visit :
brainly.com/question/18431008
#SPJ1
Give the name of the ion with 13 protons and 10 electrons
Answers
Answer:
Explanation:
aluminum
Answer: The aluminum ion
Explanation:
Arrange the following compounds in order of increasing reactivity (least reactive first.) to electrophilic aromatic substitution:.
Bromobenzene Nitrobenzene Benzene Phenol
a. Bromobenzene < Nitrobenzene < Benzene < Phenol
b. Nitrobenzene < Bromobenzene < Benzene < Phenol
c. Phenol < Benzene < Bromobenzene < Nitrobenzene
d. Nitrobenzene < Benzene < Bromobenzene < Phenol
Answers
Answer:
Nitrobenzene < Bromobenzene < Benzene < Phenol
Explanation:
Aromatic compounds undergo electrophilic aromatic substitution reaction in the presence of relevant electrophiles. Certain substituents tend to increase or decrease the tendency of an aromatic compound towards electrophilic aromatic substitution reaction.
Substituents that increase the electron density around the ring such as in phenol tends to make the ring more reactive towards electrophilic substitution. Halogens such as bromine has a -I inductive effect as well as a +M mesomeric effect.
However the -I(electron withdrawing effect) of the halogens supersedes the +M electron donation due to mesomeric effect.
Putting all these together, the order of increasing reactivity of the compounds towards electrophilic aromatic substitution is;
Nitrobenzene < Bromobenzene < Benzene < Phenol
what is meant by the term quantitative?
A) number measurement
B) words & explanation
Answers
Answer:
a) number measurement
Explanation:
relating to, measuring, or measured by the quantity of something rather than its quality.
Answer:
number and measurement
Explanation:
Quantitative is an adjective that simply means something that can be measured. For example, we can count the number of sheep on a farm or measure the gallons of milk produced by a cow.
How many moles of gold are there in 3.73 x 10^24 atoms of gold?
Answers
Answer:
6.2moles of Gold
Explanation:
To solve this problem, we are going to use the mole concept approach.
Given that;
Number of atoms of gold is 3.73 x 10²⁴ atoms
Now;
In 1 mole of any substance, we have 6.02 x 10²³ atoms;
So;
If there 6.02 x 10²³ atoms in 1 mole of any substance;
3.73 x 10²⁴ atoms will contain \(\frac{3.73 x 10^{24} }{6.02 x 10^{23} }\) = 6.2moles of Gold
Stamples of heterogeneous equilibria. FeO(s) + CO(g) = Fe(s) + CO₂(g) II. H₂(g) L₂(g) = 2HI(g) III. CO₂(g) + C(s) = 2CO(g) IV. N₂(g) 3H₂(g) + 2NH3(g) Identify I.
Answers
An example of heterogeneous equilibrium is:
I. FeO(s) + CO(g) ⇌ Fe(s) + CO₂(g)What is heterogeneous equilibrium?Heterogeneous equilibrium refers to an equilibrium state in a chemical reaction where the reactants and products exist in different physical states or phases. It occurs when substances in different phases, such as solids, liquids, and gases, are involved in a chemical reaction.
Considering the given equations:
The equation I: FeO(s) + CO(g) ⇌ Fe(s) + CO₂(g) represents a heterogeneous equilibrium.
This is because the reactants and products involve different phases (solid and gas). FeO is a solid (s), CO is a gas (g), Fe is a solid (s), and CO₂ is a gas (g). The reaction involves the conversion of a solid and a gas to another solid and a gas, and the equilibrium is established between these different phases.
Learn more about heterogenous equilibrium at: https://brainly.com/question/25257772
#SPJ1
How many atoms are in 275 grams of iron (III) hydroxide. Include units and name of atom/molecule.
Answers
To calculate the number of atoms of any compound it is necessary to use the Avogadro constant. This is a constant of proportion between the amount of matter and the number of entities that are linked to that amount. These entities can be atoms, molecules, ions, electrons, protons, neutrons. The value of the Avogrado constant is 6.022 x 10^23 mol^-1.
So to calculate the number of atoms in 275 grams of iron (III) hydroxide:
- First you need to transform grams into moles
- transform moles into atoms/molecule using Avogrado constant.
- To transform 275 grams of Fe(OH)₃ you need to use the molecular mass of Fe(OH)₃ and the following equation: mole = mass/molar mass
Molecular mass of Fe(OH)₃:
Fe - 55.85
O - 16
H - 1
(1x55.85) + (3x16) + (3x1) = 106.86 g/mol
Replace the value in the equation:
mole = 250/106.86
mole = 2.34 mole of Fe(OH)₃
- Now, let's solve the number of molecules of Fe(OH)₃ into 2.34 mole of Fe(OH)₃:
6.022 x 10^23 molecule --- 1 mol
x molecule ---- 2.34 mole
x = 1.41 x 10^24 molecules of Fe(OH)₃
Answer: There are 1.41 x 10^24 molecules of Fe(OH)₃
The name of an oxyacid has the suffix -ous acid. What is the suffix of the oxyanion?
Answers
Answer:
-ate
Explanation:
Record observations of the reaction and
products in the data table.
The brown solid that formed is
Answers
Answer:copper
Explanation:edg 2021 ;)
Answer: B.) Copper
Explanation:
The frequency of a wave can be calculated by dividing the speed of a wave by its wavelength. Calculate the frequency of a radio wave having a speed of 300,000,000 m/s and a wavelength of 0.00056 m.
Answers
Answer:
snovd vosb r
Explanation:
Which of the following atoms would have the longest de Broglie wavelength, if all have the same velocity?
A) Li
B) Na
C) Fe
D) Pb
E) Not possible to tell with given information
Answers
Answer:
Li
Explanation:
The phenomenon of wave particle duality was well established by Louis deBroglie. The wavelength associated with matter waves was related to its mass and velocity as shown below;
λ= h/mv
Where;
λ= wavelength of matter waves
m= mass of the particle
v= velocity of the particle
This implies that if the velocities of all particles are the same, the wavelength of matter waves will now depend on the mass of the particle. Hence; the wavelength of a matter wave associated with a particle is inversely proportional to the magnitude of the particle's linear momentum. The longest wavelength will then be obtained from the smallest mass of matter. Hence lithium which has the smallest mass will exhibit the longest DeBroglie wavelength
The atom that have the longest de Broglie wavelength is ; ( A ) Li
Wave particle duality is a phenomenon by de Broglie. that shows that The wavelength associated with matter waves is related to its mass and velocity .
Wave particle duality is represented as ; λ = h / mv
λ= wavelength of matter waves
m= mass of the particle
v= velocity of the particle
Given that the elements have the same velocity the atom that would have the longest de Broglie wavelength is Li
Learn more : https://brainly.com/question/21537274
A student has 2 rubber balls of the same size and weight. Ball A is still and Ball B is rolling.Ball B hits Ball A, and Ball A start moving. Why does Ball A start moving?
A). Ball A is repelled by Ball B ,
B). Ball B is attracted to Ball A ,
C). Ball B transfers energy to Ball A ,
D). Ball A transfers energy to Ball B
Answers
Answer:
C). Ball B transfers energy to Ball A
Explanation:
The motion of ball A is a because of the energy it acquires.
Ball A initially due to its position posses potential energy due to its position.
The moving ball B has kinetic energy due to the motion of this ball.
Due to the collision, momentum is transferred from the moving ball to the static one. As the momentum of the static ball changes, potential energy is converted to kinetic energy. The magnitude of the energy possessed by the static ball must be lesser than that of the moving.Answer:
c
Explanation:
when halogens are bonded to other nonmetals, the element with the higher_________ is assigned the negative number.
Answers
when halogens are bonded to other nonmetals, the element with the higher Electronegativity is assigned the negative number. Electronegativity rises from bottom to top in groups.
Electronegativity and from left to right through time. As a result, fluorine is the most electronegative element, whereas francium is one of the least. Elements having a high electronegativity are usually nonmetals or electrical insulators that function as oxidants in chemical processes. Elements having a low electronegativity, on the other hand, are often metals and strong electrical conductors that operate as reductants in chemical processes. The nucleus' positively charged protons attract the negatively charged electrons. The electronegativity or attraction rises as the number of protons in the nucleus increases. As a result, in a row of the periodic table, electronegativity rises from left to right.
learn more about Electronegativity here:
https://brainly.com/question/17762711
#SPJ4