Answers
Answer:
A. Energy can be made in factories
Explanation:
Took the quiz
Hope this helps
Related Questions
What is the temperature in kelvins of 23°C?
OA. 11.9 K
OB. 6279 K
O C. -250 K
OD. 296 K
Answers
The temperature in kelvins of 23°C is 296 K, hence option D is the correct answer.
Conversion from degree to KelvinHere's how to convert Celsius to Kelvin.
T = Tc + 273.15 ---------_We basically add a factor of 273.15 to the temperature in Celsius to get the temperature in Kelvin
T = 23 + 273.15
T = 296 K
One Celsius degree and one kelvin are precisely the same on the Kelvin scale.
Learn more about temperature measurement here:
https://brainly.com/question/777464
#SPJ1
An unknown compound contains only C , H , and O . Combustion of 5.60 g of this compound produced 13.7 g CO2 and 5.60 g H2O . What is the empirical formula of the unknown compound?
Answers
Answer:
Empirical formula of compound is C₄H₈O
Explanation:
Given data:
Mass of compound = 5.60 g
Mass of CO₂ = 13.7 g
Mass of H₂O = 5.60 g
Empirical formula of compound = ?
Solution:
Percentage of C:
13.7 g/5.60 g × 12/44× 100
2.45×0.273× 100 = 66.9%
Percentage of H:
5.60 g/ 5.60 g × 2.016/18 × 100
11.2%
Percentage of O:
(66.9% + 11.2%) - 100 = 21.9%
Grams atom of C , H, O
66.9/12 = 5.6
11.2 / 1.008 = 11.11
21.9 / 16 = 1.4
Atomic ratio:
C : H : O
5.6/1.4 : 11.11/1.4 : 1.4/1.4
4 : 8 : 1
Empirical formula:
C₄H₈O
What is the percent yield of CO2 if 46.5 grams of CO2 is recoveredfrom the reaction of 21.5 grams of C2H2 according to the reaction below?2 C2H2 + 5 O2 —> 4 CO2 + 2 H2O
Answers
The Percentage Yield is 63.9%
Hello
To solve this problem, we would calculate the theoritical yield of the reaction first.
Theoritical YieldThe theoritical yield of the reaction can be calculated using the equation of reaction.
Given that 2 moles of C₂H₂ will produce 4 moles of CO₂
This implies that
2*26g (from molar mass of C₂H₂) will produce 4*44g of CO₂
52g of C₂H₂ = 176g of CO₂
21.5g of C₂H₂ = xg of CO₂
cross multiply and solve for x
\(\begin{gathered} x=\frac{21.5\times176}{52} \\ x=72.77g \end{gathered}\)From the calculation above, the theoritical yield of CO₂ is 72.77g
Let's use this information and solve for the percentage yield.
Percentage YieldThis is the ratio between the actual yield to the theoritical yield and multiplied by 100.
Mathematically,
\(\text{percentage yield}=\frac{actual\text{ yield}}{theoritical\text{ yield }}\times100\)Data;
Actual yield = 46.5g
Theoritical yield = 72.77g
percentage yield = ?
\(\begin{gathered} \text{percentage yield}=\frac{46.5}{72.77}\times100 \\ \text{percentage yield = 63.9 \%} \end{gathered}\)From the calculations above, the Percentage Yield is 63.9%
Why is it necessary for astronauts on the International Space Station to generate and recycle oxygen?
Question 1 options:
Oxygen provides energy for the station's fuel tanks.
There is not enough oxygen in space to sustain life.
Oxygen protects the space station from radiation.
Oxygen is a by-product of human respiration.
Answers
It is necessary for astronauts on the International Space Station to generate and recycle oxygen because There is not enough oxygen in space to sustain life.
The correct answer choice is" There is not enough oxygen in space to sustain life."
In space, there is no breathable air as there is on Earth, and the amount of oxygen available is limited. Astronauts need a constant supply of oxygen to breathe and to sustain their bodily functions.
Therefore, the International Space Station has a closed-loop life support system that generates and recycles oxygen. This system converts carbon dioxide exhaled by the astronauts into oxygen through a process called electrolysis.
The generated oxygen is then supplied back into the station's atmosphere. This process helps ensure that the astronauts have a constant supply of breathable air and can survive in the space station for extended periods of time.
Therefore "There is not enough oxygen in space to sustain life." is the correct answer.
For more such questions on astronauts, click on:
https://brainly.com/question/14078735
#SPJ11
PLEASE HELP I NEED THIS DONE IN LESS THEN 2 HOURS!!! ILL GIVE BRAINLIST PLEASE HELP <3
The law of conservation of matter states that matter can be neither created nor destroyed. Your friend shows you the following chemical equation:
CaCO3 -> CaO + CO2
He says that because the oxygen atoms are split between two different molecules in the products, the equation does not support the law of conservation of matter. Is your friend right? Explain your answer. Draw a model showing the number of each type of atom in the reactants and products. Use your model to explain your answer.
Answers
The two main postulates that was given by Antoine Lavoisier are, oxygen play an important role in combustion and the other is mass of the reactant and product is conserved. Therefore, the given statement is incorrect.
What is law of conservation of mass?According to Law of conservation of mass, mass can neither be created nor be destroyed. Mass can only be transformed from one form to another. The law of conservation of mass was given by Antoine Lavoisier.
Every reaction in nature follow the law given by Antoine Lavoisier that is mass is always conserved. To see law of conservation, we need to check only number of atoms not the splitting of atoms or element. So, the given statement is incorrect.
Therefore, the given statement is incorrect.
To know more about law of conservation of mass, here:
https://brainly.com/question/28711001
#SPJ1
Enter your answer in the provided box.
Answer the following questions about the fermentation of glucose (C6H12O6, molar mass 180.2 g/mol) to ethanol (C2H6O) and CO2.
C6H12O6(s) → 2 C2H6O(l) + 2 CO2(g) ΔH = −16 kcal/mol
glucose ethanol
How many kilocalories of energy are released from 40.0 g of glucose?
kcal of energy released
Report answer to TWO significant figures.
Answers
Answer:
Explanation:
40/ 180.2 x (-16 / 1 mole glucose)=-3.6 KJ
what is the PH scale of 0.02m of hydrochloric acid
Answers
Answer:
Explanation:
The pH of 0.02 M hydrochloric acid is approximately 1.7.
THANKS
IF THE ANSWER IS CORRECT , THEN MARK ME AS BRAINLIST
To determine the pH of a hydrochloric acid solution, we need to know its concentration. You mentioned a concentration of 0.02 M (molar), which refers to 0.02 moles of hydrochloric acid dissolved in 1 liter of solution.
Hydrochloric acid (HCl) is a strong acid that dissociates completely in water, meaning all HCl molecules release their hydrogen ions (H+) into the solution. Since the concentration is given as 0.02 M, it means there are 0.02 moles of H+ ions in 1 liter of the solution.
To calculate the pH, we can use the formula:
pH = -log[H+]
In this case, [H+] represents the concentration of hydrogen ions in moles per liter. Since hydrochloric acid is a strong acid and it dissociates completely, the concentration of hydrogen ions is equal to the concentration of HCl, which is 0.02 M.
pH = -log(0.02) ≈ 1.70
Therefore, a hydrochloric acid solution with a concentration of 0.02 M would have a pH of approximately 1.70, indicating it is strongly acidic.
What type of intermolecular force will for between H2O AND CH3OH? Draw and label a picture of this bond. Explain in words how this bond forms.

Answers
Hydrogen bonding, which is unquestionably what we have, will occur from the intermolecular force between the molecules of H2O and CH3OH. Atoms trade or exchange valence electrons to create bonds.
How come we create bonds?Trust and self-esteem are developed in children and adolescents through strong emotional ties. After that, they can leave the family and establish wholesome friendships and other types of social ties. Healthy relationships consequently lower a child's chances of emotional discomfort or antisocial behaviour.
What exactly is a bonds, for example?The government of a country issues government bonds, a sort of fixed-interest bond. These bonds are thought of as low-risk investments. Examples of different kinds of government bonds include T - bills, Municipality Bond, Zero-Coupon Bonds, and others.
To know more about intermolecular visit:
https://brainly.com/question/9007693
#SPJ1
. How many moles of oxygen (O) are in 1 mole of calcium carbonate (CaCO3)?
Answers
In 1 mole of calcium carbonate, there are approximately 18.066 x 10^23 moles of oxygen.
To determine the number of moles of oxygen (O) in 1 mole of calcium carbonate (CaCO3), we need to examine the chemical formula of calcium carbonate and identify the number of oxygen atoms present.
The chemical formula of calcium carbonate is CaCO3. In this formula, we have one calcium atom (Ca), one carbon atom (C), and three oxygen atoms (O).
The subscript numbers in the formula indicate the number of atoms for each element. Therefore, we have:
1 calcium atom (Ca)
1 carbon atom (C)
3 oxygen atoms (O)
To calculate the number of moles of oxygen in 1 mole of calcium carbonate, we multiply the number of oxygen atoms (3) by the Avogadro's number, which is approximately 6.022 x 10^23 molecules/mol.
Number of moles of oxygen (O) in 1 mole of calcium carbonate (CaCO3) = 3 moles of oxygen x (6.022 x 10^23 molecules/mol)
Calculating this value, we find:
Number of moles of oxygen (O) in 1 mole of calcium carbonate (CaCO3) ≈ 18.066 x 10^23 moles of oxygen
for more such questions on calcium
https://brainly.com/question/12216405
#SPJ8
107.854 how many significant
Answers
Answer:
3
Explanation:
look after DP theres 3 digits
Consider the fructose-1,6-bisphosphatase reaction. Calculate the free energy change if the ratio of the concentrations of the products to the concentrations of the reactants is 21.321.3 and the temperature is 37.0°C37.0°C ? ΔG°′ΔG°′ for the reaction is −16.7 kJ/mol−16.7 kJ/mol .
Answers
Answer:
ΔG = -8.812 kJ/mol
Explanation:
To obtain the free energy of a reaction you can use the expression:
ΔG = ΔG° + RT ln Q
Where:
ΔG° is Standard Gibbs Free energy: -16.7kJ/mol = -16700J/mol
R is gas constant: 8.314472 J/molK
T is absolute temperature (37°C + 273.15 = 310.15K)
And Q is reaction quotient: 21.3
Replacing in the formula:
ΔG = ΔG° + RT ln Q
ΔG = -16700J/mol + 8.314472J/molK*310.15K ln 21.3
ΔG = -8812.4J/mol
ΔG = -8.812 kJ/mol
What is the mass of 6.02 x 1024 molecules of the compound HCl?
Answers
Answer:
First, we need to determine the molar mass of HCl.
The molar mass of HCl = the mass of hydrogen (1.008 g/mol) + the mass of chlorine (35.45 g/mol) = 36.45 g/mol.
Next, we can use Avogadro's number (6.02 x 10^23 molecules/mol) to convert the number of molecules to moles:
6.02 x 10^24 molecules / 6.02 x 10^23 molecules/mol = 10 moles
Finally, we can use the molar mass to convert moles to grams:
10 moles x 36.45 g/mol = 364.5 grams
Therefore, the mass of 6.02 x 10^24 molecules of HCl is 364.5 grams.
:. It means 1 mole of Hcl
:. To find the mass of HcL
no of moles = mass/ molar mass
To get the molar mass of HCL {H=1 CL=35.5}
:. H+CL = 1+ 35.5 =36.5
So we have our molar mass and number of moles now
Then we input it in the eqn
1=x/36.5
X= 36.5g of HCl
What is the molar mass of a gas if it has a density of 2.40g/L at 851.2mmHg and 2°C
Answers
Answer: The molar mass of gas is 48.4 g/mol
Explanation:
To calculate the relation of density and molar mass of a compound, we use the ideal gas equation:
\(PV=nRT\)
P = pressure = 851.2 mm Hg = 1.12 atm ( 760 mm Hg = 1atm)
V = Volume
n = number of moles
R = gas constant = 0.0821 Latm/Kmol
T = temperature = \(2^0C=(2+273)K=275K\)
Number of moles (n) can be written as:
\(n=\frac{m}{M}\)
where, m = given mass
M = molar mass
where,
\(P=\frac{mRT}{MV}\)
\(P=\frac{dRT}{M}\)
where d = density
The relation becomes:
\(M=\frac{dRT}{P}\)
Putting the values we get :
\(M=\frac{2.40g/L\times 0.0821Latm/Kmol\times 275K}{1.12atm}\)
\(M=48.4g/mol\)
Thus molar mass of gas is 48.4 g/mol
name the chemical compound

Answers
Answer:
hydrochloric acid
Explanation:
chemical compound any substance composed of identical molecules consisting of atoms
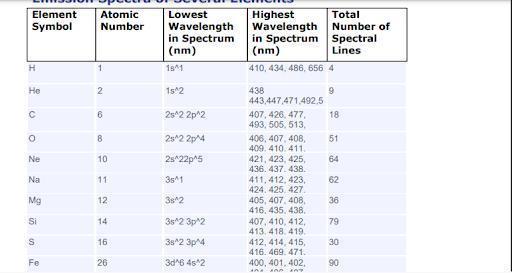
Using your data, compare atomic number with lowest and highest
wavelengths in spectrum. Is there a pattern? What might explain the
presence or absence of a pattern based on what you know about atoms,
electrons, and emission spectra?
Answers
As we continue to increase the available energy levels as shown by the principal quantum number and more electrons are added, the number of lines in the emission spectrum continues to increase.
What is a spectrum?Following the Bohr model of the atom, We could have electrons travel from a higher to a lower energy level . Thus ow leads to the emission of light of a characteristic wavelength. This line is what constitutes the emission spectrum of an atom.
If is clear from the image attached to this answer that as we continue to increase the available energy levels as shown by the principal quantum number and more electrons are added, the number of lines in the emission spectrum continues to increase.
Learn more about spectrum:brainly.com/question/3997802
#SPJ1
how does elemt's atomic mass differ from its mass number?
Answers
Answer:
Mass number is a whole number because it is the sum of number of proton and number of neutrons whereas atomic mass is fractional because it is the average relative mass of its atoms as compared with mass an atom of C-12 isotope taken as 12.
hope it helps you
What do the rows in the Periodic Table tell us?
Answers
Answer:
The rows tell you how many shells there are of the atom.
Who is the Chief of GTOCP
Answers
Answer:
???
Explanation:
What is the concentration (M) of Ch3OH a solution prepared by dissolving of CH3OH sufficient water to give exactly 230 of solution?
Answers
Answer:
1.59 M
Explanation:
What is the concentration (M) of CH₃OH a solution prepared by dissolving 11.7 g of CH₃OH sufficient water to give exactly 230 mL of solution?
Step 1: Given data
Mass of CH₃OH: 11.7 gVolume of solution: 230 mL (0.230 L)Step 2: Calculate the moles corresponding to 11.7 g of CH₃OH
The molar mass of CH₃OH is 32.04 g/mol.
11.7 g × 1 mol/32.04 g = 0.365 mol
Step 3: Calculate the molarity of the solution
M = moles of solute / liters of solution
M = 0.365 mol / 0.230 L = 1.59 M
30. What would happen if the light bulb labeled Lamp B were removed?
Switch
Lamp A
Battery
Lamp B
Lamp C
a. Lamp A and C would still light up.
b. Lamp A and C would both go out.
C. Lamp A would not light, but lamp C would.
d. Lamp A would still light, but lamp C would not.

Answers
Answer:
The answer is B.
Explanation:
The answer is be because they are all sharing the circuit, therefore they would all go out.
workers using volatile hazardous materials. Which of the following statements is true?A. A worker should feel a steady draft entering the face of the hood.B. A worker should not feel air moving around the chemical hood.C. A worker should feel a steady draft emerging from the face of the hood.
Answers
A worker should feel a steady draft emerging from the face of the hood.
What is emerging?Emerging technology is defined as the application of new and innovative technologies to existing products and services to create new ones. This form of technology is often used as a way to gain competitive advantage in the market and to stay ahead of the competition. Examples of emerging technology include artificial intelligence (AI), virtual reality (VR), robotics, blockchain, and the Internet of Things (IoT). These new technologies have the potential to revolutionize the way we do business and provide new opportunities for both individuals and businesses. As such, many companies are investing heavily in the development of these technologies in order to stay ahead of the competition and remain competitive.
To learn more about emerging.
https://brainly.com/question/6459483
#SPJ4
A student planned to make copper sulfate crystals from excess copper oxide and dilute sulfuric acid.
The equation for the reaction is:
CuO(s) + H,SO (aq) -, CuSO (aq) + H20(1)
This is the method used.
1. Add 25 cm° of dilute sulfuric acid to a conical flask.
2. Gently warm the dilute sulfuric acid.
3. Add excess copper oxide to the dilute sulfuric acid.
4. Stir the mixture.
5. Heat to evaporate all the water from the mixture.
Suggest two improvements to the method
Explain why each improvement is needed.
A student plans a method to prepare pure crystals of copper sulfate.
The student's method is:
1. Add one spatula of calcium carbonate to dilute hydrochloric acid in a beaker.
2. When the fizzing stops, heat the solution with a Bunsen burner until all the liquid is gone.
The method contains several errors and does not produce copper sulfate crystals.
Explain the improvements the student should make to the method so that pure crystals of copper sulfate are produced.

Answers
The student's method for preparing pure crystals of copper sulfate contains errors and does not produce the desired outcome.
Use copper oxide instead of calcium carbonate: The student should add copper oxide (CuO) to the hydrochloric acid instead of calcium carbonate. Copper oxide reacts with hydrochloric acid to form copper chloride, which can then be converted to copper sulfate through a subsequent reaction with sulfuric acid.
Add sulfuric acid to the copper chloride solution: After the copper chloride solution is formed, the student should add sulfuric acid to it. This reaction between copper chloride and sulfuric acid will yield copper sulfate and hydrochloric acid. The student should ensure that the correct stoichiometric ratio is maintained to maximize the yield of copper sulfate crystals.
Crystal formation: The student should allow the solution to cool slowly after the reaction with sulfuric acid. This promotes the formation of larger, well-defined copper sulfate crystals.
Filtration and drying: Once the crystals have formed, the student should filter the solution to separate the solid crystals from the remaining liquid. The filtered crystals should then be thoroughly dried to remove any remaining water, resulting in pure copper sulfate crystals.
By following these improvements, the student can obtain pure crystals of copper sulfate.
For more such questions on copper sulfate visit:
https://brainly.com/question/17439051
#SPJ8
volume reading
final: 28.5 mL
start: 7.5 mL
Total Volume: 21 mL
What is the Molarity of vinegar?
Based off the work information provided
Answers
The molarity of vinegar is 0.47368421 moles per liter.
To calculate this, we can use the following formula:
molarity = (initial_volume - total_volume_change) / final_volume
In this case, the initial volume is 7.5 mL, the total volume change is 21 mL, and the final volume is 28.5 mL. Plugging these values into the formula, we get:
molarity = (7.5 - 21) / 28.5 = -0.47368421
The negative value for molarity indicates that the solution is diluted. This is because the total volume of the solution increased by 21 mL, while the amount of solute (acetic acid) remained the same.
It is important to note that the molarity of a solution can change depending on the temperature. This is because the volume of a solution expands as it gets warmer. Therefore, it is important to measure the volume and temperature of a solution at the same time to get an accurate measurement of its molarity.
For such more questions on molarity
https://brainly.com/question/30704561
#SPJ8
What is the number of significant figures in the measurement 5.00 cubic meters? *
A. 1
B. 2
C. 3
D. 0
Answers
Carbon dioxide contributes to atmospheric warming by
Answers
write the structural formula for 2-bromo-3-chloro-4,4-dimethylpentanal
Answers
Answer:
Br-CH2-CH(CH3)2-C(Cl)H-CH(CH3)2-CHO
Explanation:
The molecule has a total of 14 carbon atoms, 13 hydrogen atoms, and 1 bromine atom. The carbon atoms are arranged in a chain with a methyl group attached to the second carbon atom, a chlorine atom attached to the third carbon atom, and two methyl groups attached to the fourth carbon atom. The fifth carbon atom has a carbonyl group attached to it.
The molecule is an aldehyde, which means that it has a carbonyl group (C=O) at the end of the chain. The carbonyl group is polar, and the oxygen atom has a partial negative charge. The hydrogen atom has a partial positive charge. This polarity makes the aldehyde group susceptible to nucleophilic attack.
The bromine and chlorine atoms are both electrophilic, which means that they have a partial positive charge. This makes them susceptible to nucleophilic attack.
The methyl groups are non-polar and do not have any significant reactivity.
The molecule is a chiral molecule, which means that it has a mirror image that is not superimposable on itself. This is because the carbon atom with the carbonyl group is attached to four different groups.
The molecule is a liquid at room temperature and has a strong odor. It is used in a variety of products, including perfumes, flavorings, and plastics.
A patient provides you a prescription for Percocet, a medication he has never taken before and his insurance company is requiring prior authorization. What steps should be taken?
Answers
To ensure insurance coverage for Percocet, it is essential to verify the patient's insurance coverage and check if prior authorization is required. If prior authorization is necessary, gather the required information, complete the authorization form, and submit it to the insurance company.
When a patient presents a prescription for a medication like Percocet, which requires prior authorization from the insurance company, several steps should be taken:
Verify Insurance Coverage: Check the patient's insurance coverage and confirm if prior authorization is required for Percocet. This can be done by contacting the insurance company or using an online portal provided by the insurer.
Review Prior Authorization Criteria: Understand the specific requirements set by the insurance company for obtaining prior authorization for Percocet. This may include documentation, medical history, and supporting evidence to justify the need for the medication.
Gather Patient Information: Collect relevant patient information, including medical records, diagnosis, and any previous treatments. This information will be used to support the prior authorization request.
Complete Prior Authorization Form: Fill out the necessary prior authorization form provided by the insurance company. Ensure that all required information is accurately entered, including the patient's details, prescriber information, and supporting documentation.
Submit the Request: Send the completed prior authorization form along with any supporting documents to the insurance company. This can be done electronically through their designated channels or by fax/mail, following their specified process.
Follow Up: Monitor the progress of the prior authorization request. Follow up with the insurance company to confirm receipt, inquire about any additional information needed, and track the status of the request.
Inform the Patient: Keep the patient informed about the prior authorization process, estimated timelines, and any potential out-of-pocket costs they may incur.
For more question on Percocet
https://brainly.com/question/28649445
#SPJ8
What is the binding energy for the nuclide 199F (atomic mass: 18.9984 amu) in MeV per nucleus?
Answers
The binding energy per nucleon for the ¹⁹F nucleon is equal to 7.786 MeV/nucleon.
What is binding energy?Binding energy can be defined as the minimum quantity of energy that is required to remove the particle from the system. Nuclear binding energy can be described as the energy required to dismantle a nucleus of an atom into free neutrons and protons.
The binding energy will be determined from the mass defect. Mass defect is calculated from the difference between the mass observed and the expected combined mass.
Given the mass of the ¹⁹F = 18.9984 a.m.u.
The mass defect for the ¹⁹F can be calculated as:
Δm = \((M _n +M_p) - M_F\)
\(\triangle m =( 9\times 1.0078 + 10 \times 1.0087 )- 18.9984\)
\(\triangle m =0.1588 \;a.m.u.\)
The binding energy for the fluorine can be calculated as:
E = Δmc²
E = 0.1588 × 931.5
E = 147.92 MeV
The binding energy per nucleon of ¹⁹F can be calculated as:
B.E.N. = 147.92/18.9984 = 7.786 MeV per nucleon
Learn more about binding energy, here:
https://brainly.com/question/10095561
#SPJ1
Which of the following molecules would you expect to have the highest boiling point?
1
O Molecule 3
O Molecule 1
O Molecule 4
O Molecule 2
2
3
OH
O
4

Answers
The highest boiling point based on the data is option 4
What is the highest boiling point?Compared to alcohols of comparable molecular weight, carboxylic acids often have higher boiling temperatures. Between the hydrogen atoms of adjacent molecules and the oxygen in the carboxyl group of carboxylic acids, strong intermolecular hydrogen bonds can develop. Because it takes more energy to break the intermolecular interactions and change the substance from a liquid to a gas during boiling, these hydrogen bonds help materials have higher boiling temperatures.
Although carboxylic acids and alcohols are both capable of forming hydrogen bonds, carboxylic acids have higher boiling temperatures due to the extra carboxyl group that they contain.
Learn more about boiling point:https://brainly.com/question/1514229
#SPJ1
Nadia runs from her house to a fiend's house that is 24 meters away. How much time she will take to reach her friend's house, knowing that Nadia's speed is 3 m/s .
Answers
Nadia will take 8 seconds to reach her friend's house.
Speed is the measure of the distance traveled by an object per unit of time. It is a scalar quantity and is typically expressed in units such as meters per second (m/s), miles per hour (mph), or kilometers per hour (km/h).
To calculate the time Nadia will take to reach her friend's house, we can use the formula;
time = distance / speed
where distance is the amount of space traveled by an object, and time is the duration of travel.
Put the values given in the problem, we have:
time = 24 meters / 3 m/s
time = 8 seconds
Therefore, Nadia will take 8 seconds.
To know more about time here
https://brainly.com/question/15356513
#SPJ1