Answers
The pressure of the gas at the new volume and temperature is the unknown.
Gas lawUsing the general gas law:
P1V1/T1 = P2V2/T2
Where
P1 = initial pressure of the gas = 1.60 atmV1 = initial volume of the gas = 546 mLT1 = initial temperature of the gas = 137 °CV2 = Final volume of the gas = 657 mLT2 = final temperature of the gas = 158 °CP2 = final pressure of the gas = not givenThus, the unknown is the final pressure, which is P2.
More on gas law can be found here: https://brainly.com/question/1190311
Related Questions
MATCH THE NAMES OF THE MICROSCOPE PARTS WITH THEIR DECRIPTIONS
Answers
The Microscope part and their right descriptions are as follows
Iris Diaphragm: A. Increases or decreases the light intensity
Objective Lens System: B. After light passes through the specimen, it next enters this lens system
Stage: C. Platform that supports a microscope slide
Adjustment Knob: D. Causes stage (or objective lens) to move upward or downward
Condenser: E. Concentrates light onto the specimen
what other parts of microscope parts and their description should you know?Other parts of a microscope and their description that you should know about includes;
Eyepiece - The lens that you look through to see the image of the specimen.
Body tube - The tube that connects the eyepiece to the objective lenses.
Arm - The part of the microscope that supports the body tube and connects it to the base.
Base - The part of the microscope that supports the arm and provides stability.
Illuminator - The light source that provides light for the microscope.
Stage clips - The clips that hold the microscope slide in place on the stage.
Revolving nosepiece - The part of the microscope that holds the objective lenses and allows them to be rotated into place.
The above answer is in response to the full question below;
Match the names of the microscope parts in column A with the descriptions in column B. Place the letter of your choice in the space provided.
1. Iris diaphram
2. Objective lens system
3. Stage
4. Adjustment knob
5. Condenser
Increases or decreases the light intensity
2. After light passes through the specimen, it next enters this lens system
3. Platform that supports a microscope slide
4. Causes stage (or objective lens) to move upward or downward
5. Concentrates light onto the specimen
Find more exercises on Microscope;
https://brainly.com/question/1869322
#SPJ1
The center of gravity is the same for all passenger vehicles.
Answers
The center of gravity is the same for all passenger vehicles no it is different
The center gravity is the point through which the force of gravity acts on an object or system then in the mechanics problem the gravitational field is assumed to be uniform and the center of gravity is then in exactly the same position as the center of mass and the the center of gravity is the same for all passenger vehicles no it is different because the thermotical point where the sum of all of the masses of each of its individual components effectively act and the center of gravity is low then the stability of vehicle and passenger are more
Know more about vehicles
https://brainly.com/question/20662235
#SPJ1
Of the following regions of the electromagnetic spectrum, which one has the shortest wavelength?
a.
gamma rays
b.
infrared
c.
radio waves
d.
X rays
e.
microwaves
f.
ultraviolet
Answers
Answer:
A ---->gamma ray
Explanation:
Gamma rays have the highest frequencies among all electromagnetic waves and therefore have the shortest wavelengths.
What is the atomic number for an element whose mass number is 78,
which contains 30 neutrons per atom?
Answers
Answer:
protons = atomic numbermass number = protons + neutronsatomic number = mass number - neutrons = 78-38 = 40
Explanation:
The atomic number of an element represents the number of protons in its nucleus.
To determine the atomic number, subtract the number of neutrons from the mass number.
In this case, the mass number is 78, and there are 30 neutrons per atom. So, atomic number = mass number - number of neutrons = 78 - 30 = 48.
Therefore, the element with a mass number of 78 and 30 neutrons per atom has an atomic number of 48. The element with atomic number 48 is cadmium (Cd), which means it has 48 protons in its nucleus.
Know more about protons:
https://brainly.com/question/29248303
#SPJ3
help asap 100pts -
What mass of H2 is needed to react with 8.75 g of O2 according to the following equation:
O2(g) + 4H2(g) → 2H2O(g)?
O 0.547 g H₂
O 2.21 g H₂
O 4.38 g H₂
O 17.5 g H₂
Answers
To solve this problem, we need to use stoichiometry and the balanced equation for the reaction. From the balanced equation, we see that 4 moles of H2 are required to react with 1 mole of O2 to produce 2 moles of H2O.
1 mole O2 = 32 g O2
8.75 g O2 = 8.75 g / 32 g/mol = 0.2734 moles O2
Using the stoichiometry of the balanced equation, we can determine the moles of H2 needed to react with the given moles of O2:
0.2734 moles O2 × 4 moles H2 / 1 mole O2 = 1.0936 moles H2
Finally, we can convert the moles of H2 to grams using the molar mass of H2:
1.0936 moles H2 × 2 g/mol H2 = 2.1872 g H2
Therefore, the mass of H2 needed to react with 8.75 g of O2 is approximately 2.19 g H2 (to two significant figures).
So, the closest option is O 2.21 g H₂.
2FeCl+ CL - 2 FeCl3
Given: 175.98 grams Cl2 reacted
Wanted: grams of FeCl reacted?
Answers
Answer:
Explanation:
The balanced chemical equation for the reaction between FeCl and Cl2 is:
2FeCl + Cl2 → 2FeCl3
From the equation, we can see that 1 mole of Cl2 reacts with 2 moles of FeCl. To find the amount of FeCl that reacted when 175.98 grams of Cl2 reacted, we need to follow a few steps:
Calculate the number of moles of Cl2 that reacted:
mass of Cl2 / molar mass of Cl2 = 175.98 g / 70.906 g/mol = 2.479 mol Cl2
Use the mole ratio from the balanced equation to find the number of moles of FeCl that reacted:
2 moles FeCl / 1 mole Cl2 = x moles FeCl / 2.479 moles Cl2
x moles FeCl = 2 moles FeCl / 1 mole Cl2 x 2.479 moles Cl2 = 4.958 moles FeCl
Calculate the mass of FeCl that reacted:
mass of FeCl = moles of FeCl x molar mass of FeCl = 4.958 moles x (55.845 + 35.453) g/mol = 340.3 g
Therefore, 340.3 grams of FeCl reacted when 175.98 grams of Cl2 reacted.
One kilometre equals to what centimeter
Answers
Answer:
100,000
Explanation:
1 kilometer equals 100,000 centimeters
You multiply the length value by 100,000
Answer:
One kilometre is 100,000 centimeters
Explanation:
Which of the following could be the cause of a volume increase in a container?temperature is increasedpressure is decreasedpressure is increasedtemperature is decreased
Answers
Answer
Pressure is decreased
Explanation
As the pressure on a gas increases, the volume of the gas decreases because the gas particles are forced closer together. Conversely, as the pressure on a gas decreases, the gas volume increases because the gas particles can now move farther apart.
Which of these would be considered a basic nucleophile for reaction with an epoxide? (Select all that apply) O HBr O H20, H2SO4 O LiAlH4 O RMgBr O HCN D O CH3ONa, CH3OH O NaOH, H20O HCI O CH3OH, H2SO4 O KCN, DMF O CH3CC- (alkynyl anion)
Answers
The following nucleophiles would be considered basic for reaction with an epoxide: NaOH, H2O, CH3ONa, CH3OH, LiAlH4, RMgBr and CH3CC- (alkynyl anion).
Which are more reactive- acidic or basic nucleophiles?Basic nucleophiles are typically more reactive towards epoxides than acidic nucleophiles due to their electron-rich nature, which allows them to attack the highly electrophilic carbon centre of the epoxide ring. Acidic nucleophiles are species that can donate electrons to form a new bond with an electron-deficient center, such as a carbocation or an electrophilic carbon atom.
What do these basic nucleophiles do?These nucleophiles can attack the carbon centre of the epoxide and open the ring to form a new alkoxide or alkyne product, depending on the specific conditions and reagents used.
To learn more about nucleophiles, visit here:
https://brainly.com/question/28325919
#SPJ1
The smallest possible particle of an element is a(n) .
Answers
Answer: Atoms are the smallest particles of an element.
a forest is a habitat for many living things. what happens to these different living things
Answers
Forest is a habitat for many plants and animals because it provides a suitable environment for them.
Forest is a habitat for many plant life and animals as it offers a appropriate environment for them. As an instance, neem, Sheesham, Palash, tiger, porcupine, elephants, jackal and so forth. The ground beneath the tree of the forest is the forest floor.
It causes habitat destruction, accelerated chance of predation, reduced food availability, and lots greater. As a result, some animals lose their homes, others lose meals assets – and ultimately, many lose their lives. In reality, deforestation is one of the essential causes of extinction.
Weather trade also alters the life cycles of flora and animals. as an instance, as temperatures get warmer, many plant life are starting to develop and bloom earlier inside the spring and live to tell the tale longer into the fall. A few animals are waking from hibernation sooner or migrating at different instances, too.
Learn more about habitat here:- https://brainly.com/question/23780904
#SPJ9
i need help
can anyone help me

Answers

what is the molar mass of PbCO₄
Answers
Answer:
283.211
Explanation:

What mass of Fe(OH)3 will be obtained when 100. mL of 0.240 M FeCl3 is mixed with 200. mL of 0.182 M NaOH?
Answers
Answer:
648.68 mg
Explanation:
The reaction that takes place is:
FeCl₃ + 3NaOH → Fe(OH)₃ + 3NaClFirst we calculate how many moles of each reactant were added, using the given volumes and concentrations:
FeCl₃ ⇒ 100 mL * 0.240 M = 24 mmol FeCl₃NaOH ⇒ 100 mL * 0.182 M = 18.2 mmol NaOH24 mmol of FeCl₃ would react completely with (24 * 3) 72 mmol of NaOH. There are not as many NaOH mmoles, so NaOH is the limiting reactant.
Now we calculate how many moles of Fe(OH)₃ are formed, using the moles of the limiting reactant:
18.2 mmol NaOH * \(\frac{1mmolFe(OH)_3}{3mmolNaOH}\) = 6.07 mmol Fe(OH)₃Finally we convert 6.07 mmol Fe(OH)₃ to grams, using its molar mass:
6.07 mmol Fe(OH)₃ * 106.867 mg/mmol = 648.68 mgThe 129.6 g mass of Fe(OH)₃ will be obtained.
How we calculate mass or weight from moles?Weight or mass of any substance can be calculated from mole as:
n = W/M.
Given chemical reaction is:
FeCl₃ + 3NaOH → Fe(OH)₃ + 3NaCl
According to the question:
Concentration of FeCl₃ = 0.240 M
Volume of FeCl₃ = 100 mL
Concentration of NaOH = 0.182 M
Volume of NaOH = 200 mL
Moles can be calculated by using the formula M = n/V mole/L 0r M = n×1000/V g/mL,
Moles of FeCl₃ = 0.240 × 100 = 0.024 mole
Moles of NaOH = 0.182 × 200 = 3.64 mole
From the stoichiometry of the reaction, it is clear that 3 mole of NaOH i.e. 109.2 moles of NaOH reacts with 1 mole of FeCl₃ to form product. So here NaOH is the limiting agent and responsible for the formation of product. Now we calculate how many moles of Fe(OH)₃ are formed, using the moles of the limiting reactant as follow:
Moles of Fe(OH)₃ = 1/3 × ( 3.64) = 1.21 mole
Finally we convert 12.1 mole Fe(OH)₃ to grams, using its molar mass:
W = 1.21 mole × 106.87 g/mole = 129.6 g
Hence, 129.6 gram of Fe(OH)₃ will be obtained.
To know more about moles, visit the below link:
https://brainly.com/question/14464650
ion
Р
Question 6
1321 ✪
9 words
Consider the reaction 3X + 2Y→ 5C + 4D
How many moles of C can be synthesized from 33.0 moles of Y?
Round your answer to a whole number.
1 pts
Answers
Answer:
83
Explanation:
3X + 2Y → 5C + 4D
2 moles of Y will produce 5 moles of C
33.0 moles of Y will produce: 33.0 x 5/2 = 82.5 or 83 moles of C
Which two particles are found in the nucleus of an atom?
neutrons and electrons
protons and electrons
protons and neutrons
neutrons and atoms
Answers
Answer:
C.) protons and neutrons
Explanation:
Most atoms contain proton(s), neutron(s), and electron(s). Within the nucleus of an atom, there are protons and neutrons. Electrons are located outside of the nucleus.
Which of the following is true for the quantum mechanical atomic model?
A. Atoms absorb or emit electrons from the nucleus when they interact with electromagnetic radiation.
B. Every atom absorbs all wavelengths of light energy or electromagnetic radiation.
C. Electrons give off electromagnetic radiation when they jump from a high to a low energy level.
D. Electrons are perfectly evenly distributed throughout the atom.
Answers
The true statement for the quantum mechanical atomic model is that Electrons give off electromagnetic radiation when they jump from a high to a low energy level.
Option c is correct.
What is e quantum mechanical atomic model?The quantum mechanical atomic model, also known as the wave mechanical model, describes the behavior of electrons in atoms as waves.
This model described that only electrons can exist only in certain discrete energy levels around the nucleus. When an electron absorbs energy, it moves to a higher energy level.
In other words, when it loses energy, it moves to a lower energy level.
Learn more about quantum mechanical atomic model at:
https://brainly.com/question/3504937
#SPJ1
How to answer this? (picture attached)

Answers
Explanation:
add HCl to it.
it will become 1,1-dichloro ethane which is a monomer.
hope this helps.
Sulphur +_________ gives ➙ sulphur oxide
Answers
Answer:
Sulphur + oxygen gives ➙ Sulphur oxide
Explanation:
Sulfur oxide is a compound that consists of sulfur and oxygen molecules. I hope this helps!
The fluoride ion is the conjugate base of the weak acid hydrofluoric acid. The value of Kb for F-, is 1.39×10-11. Write the equation for the reaction that goes with this equilibrium constant.
Answers
Answer:
F⁻(aq) + H₂O(l) ⇄ HF(aq) + OH⁻(aq)
Explanation:
According to Brönsted-Lowry acid-base theory, an acid is a substance that donates H⁺ ions. In this sense, hydrofluoric acid is an acid according to the following equation.
HF(aq) + H₂O(l) ⇄ F⁻(aq) + H₃O⁺(aq)
According to Brönsted-Lowry acid-base theory, a base is a substance that accepts H⁺ ions. In this sense, the fluoride ion is a base according to the following equation.
F⁻(aq) + H₂O(l) ⇄ HF(aq) + OH⁻(aq)
The equilibrium constant for this reaction is Kb = 1.39 × 10⁻¹¹.
5 Questions, don't need steps, round using SIG FIGS (significant figures)
Honors Chem (oC = Celcius)
1. Calculate the volume, in L, of oxygen gas produced at 1.05 atm and 25.0 oC by the decomposition of 10.5 g of potassium chlorate.
2 KClO3(s) --> 2 KCl(s) + 3 O2(g)
2. If 8.5 g of CaF2 is reacted with an excess of H2SO4, what volume, in L, of gaseous HF will be produced at a temperature of 19 oC and a pressure of 855 mm Hg?
CaF2(s) + H2SO4(aq) --> 2 HF(g) + CaSO4(s)
3. Joseph Priestly decomposed mercury(II) oxide, HgO, to produce oxygen in some of his early experiments in which he discovered oxygen. If 125 g of HgO is decomposed, what pressure, in atm, of gaseous O2 will be produced at a temperature of 121oC and a volume of 9.61 L?
2 HgO(s) --> 2 Hg(l) + O2(g)
4. If 25.0 g of sodium borohydride, NaBH4, is reacted with an excess of H2SO4, what pressure, in mm Hg, of gaseous B2H6 will be produced at a temperature of 16 oC and a volume of 8.10 L?
2 NaBH4(s) + H2SO4(aq) --> B2H6(g) + 2 H2(g) + Na2SO4(aq)
5. Sodium bicarbonate reacts with phosphoric acid to form sodium phosphate, water, and carbon dioxide gas. If 24.3 L of carbon dioxide gas is formed at 273 K and 1.00 atm, how many grams of phosphoric acid reacted?
3 NaHCO3(aq) + H3PO4(s) --> Na3PO4(aq) + 3 H2O(l) + 3 CO2(g)
Answers
1) Volume of oxygen gas produced is 3.19 L ; 2) Volume of gaseous HF produced is 4.92 L ; 3) Pressure of gaseous O₂ produced is 10.0 atm. ; 4) Pressure of gaseous B₂H₆ produced is 6292 mm Hg.5) 34.6 grams of phosphoric acid reacted.
What is molar mass?Molar mass can be defined the mass of one mole of substance.
1.) Molar mass of potassium chlorate (KClO₃) is: 39.098 g/mol + 35.453 g/mol + 3 * 15.999 g/mol = 122.549 g/mol
10.5 g KClO₃ * (1 mol KClO₃ / 122.549 g KClO₃) * (3 mol O₂ / 2 mol KClO₃) = 0.1281 mol O₂
As V = (nRT) / P
= (0.1281 * 0.08206 * (25.0 + 273.15) ) / 1.05
So V = 3.19 L
Therefore, the volume of oxygen gas produced is 3.19 L.
2.) Molar mass of calcium fluoride (CaF₂) is: 40.078 g/mol + 2 * 18.998 g/mol = 78.076 g/mol
8.5 g CaF₂ * (1 mol CaF₂ / 78.076 g CaF₂) * (2 mol HF / 1 mol CaF₂) = 0.2171 mol HF
V = (0.2171 * 0.08206 / * 292.15 ) / 1.124
V = 4.92 L
Therefore, volume of gaseous HF produced is 4.92 L.
3.) Molar mass of HgO = 200.59 g/mol
125 g HgO = 125/200.59 = 0.623 mol HgO
From the balanced equation, 2 moles of HgO produces 1 mole of O₂
Therefore, 0.623 mol HgO produces 0.3115 mol O₂
As P = nRT/V
= (0.3115 mol)(0.08206 )(394.15 )/(9.61 )
P = 10.0 atm
Therefore, pressure of gaseous O₂ produced is 10.0 atm.
4. Molar mass of NaBH₄ = 37.83 g/mol
25.0 g NaBH₄ = 25.0/37.83 = 0.661 mol NaBH4
From the balanced equation, 2 moles of NaBH₄ produces 1 mole of B₂H₆
Therefore, 0.661 mol NaBH₄ produces 0.3305 mol B₂H₆
As P = nRT/V
= (0.3305 )(0.08206 )(289.15 )/(8.10 )
P = 8.29 atm
8.29 atm = 6292 mm Hg
Therefore, pressure of gaseous B₂H₆ produced is 6292 mm Hg.
5.) As n = PV/RT
n = (1.00 )(24.3 )/(0.0821 )(273 ) = 1.06 mol CO₂
1.06 mol CO₂ × (1 mol H₃PO₄/3 mol CO2) = 0.353 mol H₃PO₄
Molar mass of H₃PO₄ is 98.0 g/mol; so 0.353 mol H₃PO₄ × (98.0 g H₃PO₄/mol) = 34.6 g H₃PO₄
Therefore, 34.6 grams of phosphoric acid reacted.
To know more about molar mass, refer
https://brainly.com/question/837939
#SPJ1
convert 3.01•10^23 molecules of C2H6 to moles.
Answers
Answer:
The answer is 0.5 molesExplanation:
To find the number of moles in a substance given it's number of entities we use the formula
\(n = \frac{N}{L} \\ \)
where n is the number of moles
N is the number of entities
L is the Avogadro's constant which is
6.02 × 10²³ entities
From the question we have
\(n = \frac{3.01 \times {10}^{23} }{6.02 \times {10}^{23} } = \frac{3.01}{6.02} \\ \)
We have the final answer as
0.5 molesHope this helps you
Which scientific term names rocks formed from magma?
A. Intrusive
B. Lava
C. Gabbro
D. Extrusive
Answers
Answer:
A Intrusive
Explanation:
I go to K12 and they say that's the answer
How many total atoms are in 0.940 g of P2O5?
Answers
Hope it is Helpful to you
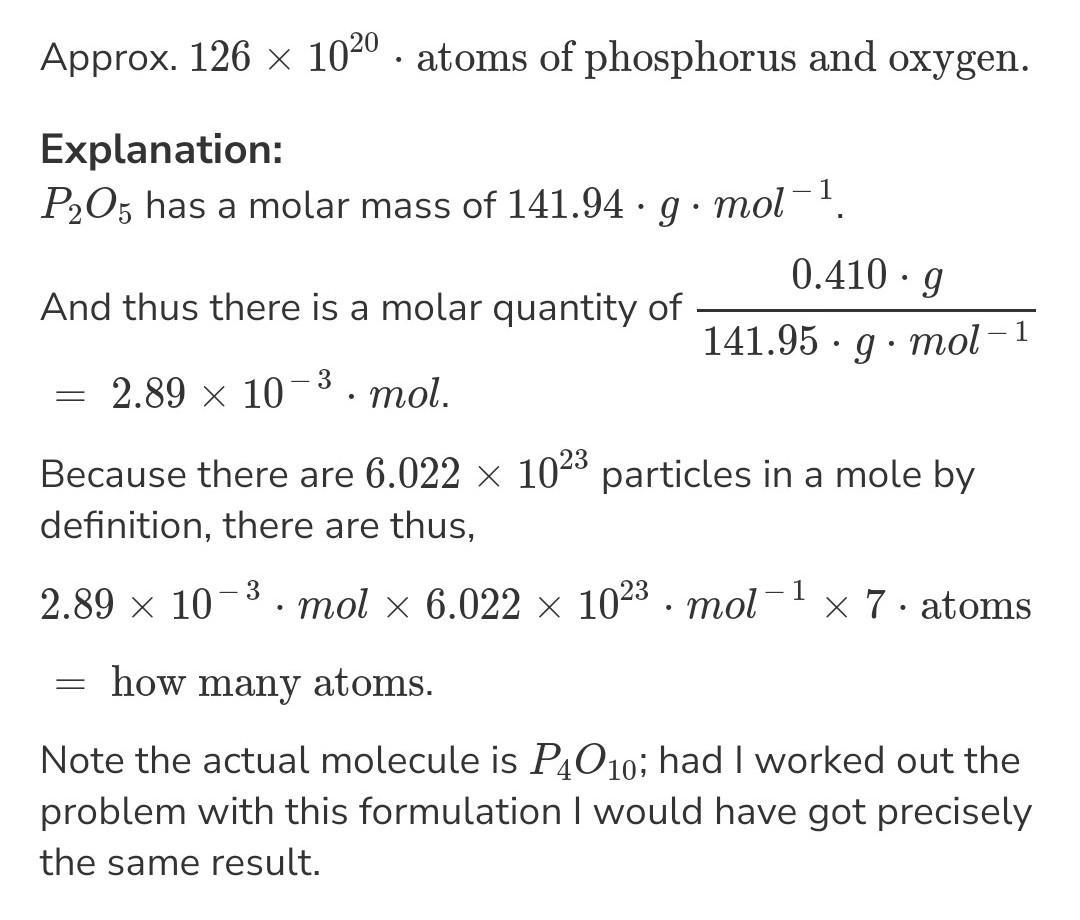
The number of atoms in the 0.940 grams P₂O₅ is equal to 3.4 × 10²¹ atoms.
What is Avogadro's number?Avogadro’s number is the number of particles in one mole of any substance or element. Generally, these entities can be atoms, molecules, ions, protons, or electrons, depending upon the kind of chemical reaction or reactant and product.
The Avogadro’s constant is experimentally found approximately equal to 6.022 × 10²³ mol⁻¹.
Given, the amount of phosphorous oxide, P₂O₅ = 0.940 g
The molecular mass of the phosphorous oxide, P₂O₅ = 141.95 g/mol
It means that 141.95 g of P₂O₅ contains atoms = 6.022 × 10²³
Then 0.940 g of P₂O₅ will contain atoms = (0.940/141.95) × 6.022 × 10²³
= 0.034 × 10²³ atoms
= 3.4 × 10²¹ atoms
Therefore, the atoms in the 0.940 grams P₂O₅ are 3.4 × 10²¹ atoms.
Learn more about Avogadro's number, here:
brainly.com/question/11907018
#SPJ2
(a) Describe the process by which Nitrogen is obtained from air on a large scale
Answers
The element nitrogen exists as a gas and is obtained from air on a large scale by fractional distillation of air.
What is an element?An element is defined as a substance which cannot be broken down further into any other substance. Each element is made up of its own type of atom. Due to this reason all elements are different from one another.
Elements can be classified as metals and non-metals. Metals are shiny and conduct electricity and are all solids at room temperature except mercury. Non-metals do not conduct electricity and are mostly gases at room temperature except carbon and sulfur.
The number of protons in the nucleus is the defining property of an element and is related to the atomic number.All atoms with same atomic number are atoms of same element.
Learn more about element,here:
https://brainly.com/question/14347616
#SPJ2
If you need 3L of NH3 how many grams of N2 are required?3H2(g) + N2(g) ==> 2NH3(g)
Answers
Answer
1.88 grams
Explanation
Given:
3H2(g) + N2(g) ----> 2NH3(g)
Volume of NH3(g) = 3 L
What to find:
The grams of N2(g) required.
Step-by-step solution:
From this balanced equation, 3 moles of H2(g) react with 1 mole N2(g) to produce 2 moles NH3(g).
That can also mean 3 liters of H2(g) reacts with 1 liter N2(g) to produce 2 liters of NH3(g).
Thus, if it takes 1 liter of N2(g) to produce 2 liters of NH3(g),
Then it will take x liters of N2(g) to produce 3 liters of NH3(g)
To get x, cross multiply and divide both sides by 2 liters NH3(g):
\(x=\frac{1\text{ }liter\text{ }N_2(g)\times3\text{ }liters\text{ }NH_3(g)}{2\text{ }liters\text{ }NH_3(g)}=1.5\text{ }liters\text{ }N_2(g)\)1.5 liters of N2(g) are required to produce 3 L of NH3(g).
The final step is to convert 1.5 liters of N2(g) to grams.
1 mole of N2(g) occupies 22.4 L at STP
Note: 1 mole of N2(g) = 28.01 g
Thus, if 28.01 grams N2(g) occupies 22.4 L at STP
Then the mass of N2(g) in 1.5 L of N2(g) is:
\(\frac{1.5\text{ }L\times28.01\text{ }grams}{22.4\text{ }L}=1.88\text{ }grams\)Therefore, the grams of N2(g) required to 3L of NH3(g) is 1.88 grams
Which of these half-reactions represents reduction?
I. Fe2+ → Fe3+
II. Cr2O72- → Cr3+
III. MnO4- → Mn2+
Answers
Answer: The half-reactions represents reduction are as follows.
\(Cr_{2}O^{2-}_{7} \rightarrow Cr^{3+}\)\(MnO^{-}_{4} \rightarrow Mn^{2+}\)Explanation:
A half-reaction where addition of electrons take place or a reaction where decrease in oxidation state of an element takes place is called reduction-half reaction.
For example, the oxidation state of Cr in \(Cr_{2}O^{2-}_{7}\) is +6 which is getting converted into +3, that is, decrease in oxidation state is taking place as follows.
\(Cr_{2}O^{2-}_{7} + 3 e^{-} \rightarrow Cr^{3+}\)
Similarly, oxidation state of Mn in \(MnO^{-}_{4}\) is +7 which is getting converted into +2, that is, decrease in oxidation state is taking place as follows.
\(MnO^{2-}_{4} + 5 e^{-} \rightarrow Mn^{2+}\)
Thus, we can conclude that half-reactions represents reduction are as follows.
\(Cr_{2}O^{2-}_{7} \rightarrow Cr^{3+}\)\(MnO^{-}_{4} \rightarrow Mn^{2+}\)Starting with 0.3500 mol CO(g) and 0.05500 mol COCl2(g) in a 3.050 L flask at 668 K, how many moles of CI2(g) will be present at equilibrium?
CO(g) + Cl2(g)》COCl2(g)
Kc= 1.2 x 10^3 at 668 K
Answers
At equilibrium, the number of moles of \(Cl_2\) (g) will be 0.2025 mol.
1: Write the balanced chemical equation:
\(C_O\)(g) + \(Cl_2\)(g) ⟶ \(C_OCl_2\)(g)
2: Set up an ICE table to track the changes in moles of the substances involved in the reaction.
Initial:
\(C_O\)(g) = 0.3500 mol
\(Cl_2\)(g) = 0.05500 mol
\(C_OCl_2\)(g) = 0 mol
Change:
\(C_O\)(g) = -x
\(Cl_2\)(g) = -x
\(C_OCl_2\)(g) = +x
Equilibrium:
\(C_O\)(g) = 0.3500 - x mol
\(Cl_2\)(g) = 0.05500 - x mol
\(C_OCl_2\)(g) = x mol
3: Write the expression for the equilibrium constant (Kc) using the concentrations of the species involved:
Kc = [\(C_OCl_2\)(g)] / [\(C_O\)(g)] * [\(Cl_2\)(g)]
4: Substitute the given equilibrium constant (Kc) value into the expression:
1.2 x \(10^3\) = x / (0.3500 - x) * (0.05500 - x)
5: Solve the equation for x. Rearrange the equation to obtain a quadratic equation:
1.2 x \(10^3\) * (0.3500 - x) * (0.05500 - x) = x
6: Simplify and solve the quadratic equation. This can be done by multiplying out the terms, rearranging the equation to standard quadratic form, and then using the quadratic formula.
7: After solving the quadratic equation, you will find two possible values for x. However, since the number of moles cannot be negative, we discard the negative solution.
8: The positive value of x represents the number of moles of \(Cl_2\)(g) at equilibrium. Substitute the value of x into the expression for \(Cl_2\)(g):
\(Cl_2\)(g) = 0.05500 - x
9: Calculate the value of \(Cl_2\)(g) at equilibrium:
\(Cl_2\)(g) = 0.05500 - x
\(Cl_2\)(g) = 0.05500 - (positive value of x)
10: Calculate the final value of \(Cl_2\) (g) at equilibrium to get the answer.
Therefore, at equilibrium, the number of moles of \(Cl_2\) (g) will be 0.2025 mol.
For more such questions on equilibrium, click on:
https://brainly.com/question/517289
#SPJ8
Answer the following questions about the solubility of CoCO3(s). The value of Ksp for CoCO3(s) is 1.0 × 10^−10.
A. Calculate the value of [Co2+] in a saturated solution of CoCO3 in distilled water.
B. If 0.10 M of Co2+ is already present in distilled water, calculate the molar solubility of CoCO3(s).
C. Explain why CoCO3 is less soluble in distilled water that already contains Co2+
Answers
Answer:
Attached picture.
Explanation:
(1) Ksp equals the product of [Co 2+][CO3 2-]. CoCO3 is excluded from the equilibrium expression because it is a pure solid. The mole ratio of Co 2+ and CO3 2- is 1:1 so their molar solubilities are the same.
(2) There is an initial concentration of 0.10 M Co 2+ so write that in the "I" row for Co 2+ on the ICE table. When you find the zeros of the quadratic when solving for "s", take the positive value rather than the negative value because concentration cannot be negative.
(3) Extra products will cause the equilibrium to consume products and form reactants. So the reverse reaction will occur faster than the forward reaction. More products mean an increased Q value compared to K, since the numerator of \(K = \frac{[products]}{[reactants]}\) increases.

Lab 2: paper chromatography of organic dyes
Picture of questions below.

Answers
Answer:
The three primary colors used when mixing dyes or paints are red, yellow, and blue. Other colors are often a mixture of these three colors. Try running a chromatography test again with non-primary-color markers, like purple, brown, and orange.
Explanation:
Mixtures that are suitable for separation by chromatography include inks, dyes and colouring agents in food. ... As the solvent soaks up the paper, it carries the mixtures with it. Different components of the mixture will move at different rates. This separates the mixture out.